

Stryker is a proud supporter of Congress of Neurological Surgeons.
As a market leader, our innovative access, intervention and closure solutions for neurosurgery support efficiency and improved surgical outcomes for the surgeons and patients we serve. We invite you to learn more about our cranial product lines below or visit our expanded neurotechnology offerings at stryker.com/neurotechnology.
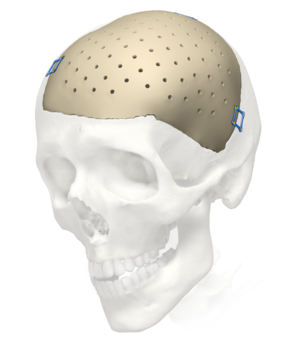
Cranial iD
Precise fit. Excellent strength.
PEEK and MEDPOR material options
Our iD Solutions, Cranial iD implants allow you to address your patient’s desire for complete restoration and aesthetic results. Available in the material of your choice including MEDPOR and PEEK, each implant is uniquely designed to fit the bony void and individual anthropometry of your patient. You drive the artistry of each implant through a design session with one of our Design Engineers. The end result is an exceptional anatomical fit and contour. Further your ability to address patient needs now and in the future with our Pterional PLUS implant. Our exclusive implant allows you the opportunity to prevent and correct persistent temporal hollowing (PTH) in your patients. Pterional PLUS can help patients regain confidence helping them to look and feel like themselves again.
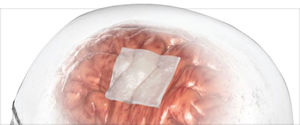
DuraMatrix Portfolio
Restore with confidence.
To ensure safety and quality, products are comprised of Type I collagen and intact dermis tissue derived from a safe, reliable bovine source.
DirectInject
Ready when you are.
A self-setting, calcium phosphate cement intended to repair neurosurgical burr holes, contiguous craniotomy cuts and other cranial defects not intrinsic to the stability of the bony structure. It is also intended for augmentation or restoration of bony contour in the craniofacial skeleton to include the cranial and zygomatic bones. DirectInject is intended to repair cranial defects with a surface area of 4 cm2 or less and is indicated for patients in whom skeletal growth is complete. You can use it in patients with surgically created bone defects.

Delta
Balanced resorption. Trusted strength.
A resorbable plating system designed for use in the fixation of bone of the cranialfacial and midfacial skeleton affected by trauma or for reconstruction.
MEDPOR Neuro
Proven1. Adaptable. Comprehensive.
Since 1985, MEDPOR porous polyethylene implants have provided you with a range of off-the-shelf, biocompatible implant options for your aesthetic, reconstruction and augmentation needs. Our iD Solutions portfolio offers customized implants made with MEDPOR biomaterial for your patient specific needs.

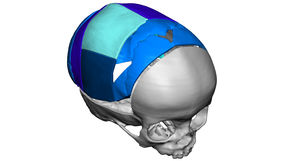
VSP Cranial
Mark. Position. Reconstruct.
Working in collaboration with 3D Systems, VSP Cranial represents a major leap forward in virtual planning of orthognathic surgical procedures. Our sales representatives are with you through every step of the process.
Stryker Corporation or its affiliates own, use, or have applied for the following trademarks or service marks: CranialMap, E-SEP, Hybrid MMF, MEDPOR, RemB, SMARTLock, SONOPET, Stryker NAV3i and Stryker. All other trademarks are trademarks of their respective owners or holders.
1: Liu JK, Gotfried ON, Cole CD, Dougherty,WR, Couldwell WT, “MEDPOR Porous Polyethylene Implant for Cranioplasty and Skull Base Reconstruction” Neurosurgery [April 2004]
CMF-WC-31_Rev. None_15010
