MEDPOR®
Oculoplastic surgery
Overview
MEDPOR has been a trusted name in the industry since 1985, with hundreds of thousands of procedures performed, and hundreds of published clinical reports in reconstructive, cranial, oculoplastic, and cosmetic applications.
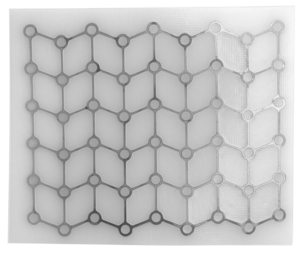
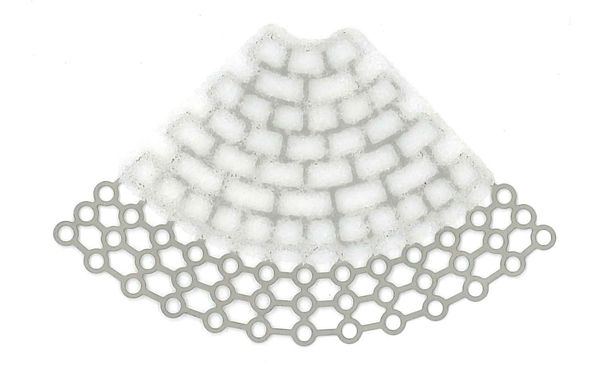
Our MEDPOR product line provides you an array of porous polyethylene solutions for your reconstruction and augmentation needs. We understand that bio-compatibility characteristics of implants are paramount to help surgeons achieve positive patient outcomes. The omni-directional pore structure of our polyethylene implants may increase implant acceptance by allowing the patient‘s native tissue to integrate with the implant. In addition to our comprehensive line of stock MEDPOR implants, we offer CT-based patient-specific implants, putting the implant design in your hands.
Proven. Adaptable. Comprehensive.
Features and benefits

Proven material
More than 400,000 procedures have been performed with MEDPOR Biomaterial, with more than 350 published clinical reports in cranial, reconstructive, oculoplastic and cosmetic applications.
Comprehensive portfolio
Wide variety of facial implants to fit patient and surgeon reconstructive and augmentation needs.

MEDPOR TITAN
Combines high-density polyethylene sheets and titanium mesh in a single implant for increased flexibility, shape retention, radiographic visualization and strength.1

Adaptable
If desired, MEDPOR material may be cut and shaped to fit patient and surgeon needs.
Sterile
Implants packaged sterile and ready to use. They can be stored in the hospital or office to have on hand as needed.
Clinical evidence
Objective
To determine the safety and efficacy of using porous high-density polyethylene (PHDPE) in the repair of orbital defects.
Design
Retrospective case series.
Setting
Academic tertiary care trauma center. Patients One hundred seventy patients with orbital defects requiring surgical repair. Intervention Orbital defect repair with PHDPE. Main Outcome Measure Our review documents surgical results and complications associated with the use of PHDPE.
Results
There was a 6.4% complication rate associated with the use of PHDPE. The infection rate was 1.8%. The persistent orbital malposition rate was 3.5%. The extrusion rate was 0%.
Conclusions
This report represents the largest case series in the literature using PHDPE for orbital reconstructions. The use of PHDPE resulted in a low complication rate and excellent functional and cosmetic reconstructive results. Because of our success with the use of PHDPE, we have changed our clinical practice to minimize the use of autologous graft material, thereby eliminating donor site morbidity in cases involving orbital reconstruction.
References:
1: Holck, D., Foster J., and Dahl T., “Custom Shaped Porous Polyethylene-Titanium Mesh Orbital Implants for Internal Orbital Floor/Medial- Wall Fracture Repair” ASOPRS 37th Annual Fall Scientific Syllabus, pp190, November 15-16, 2006
CMF-WC-53_Rev. None_18992



.jpg)