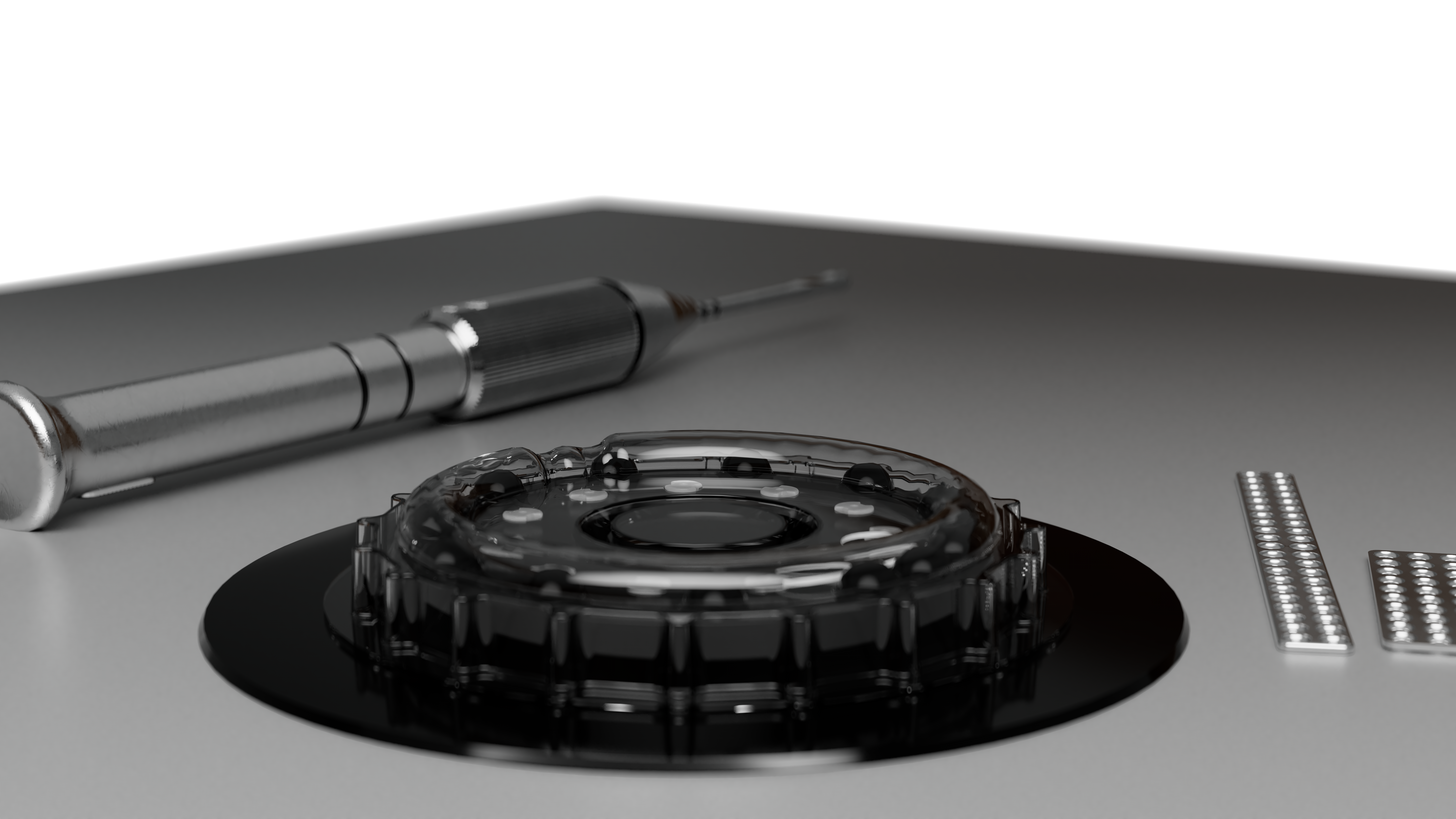
Delta DualStart Overview
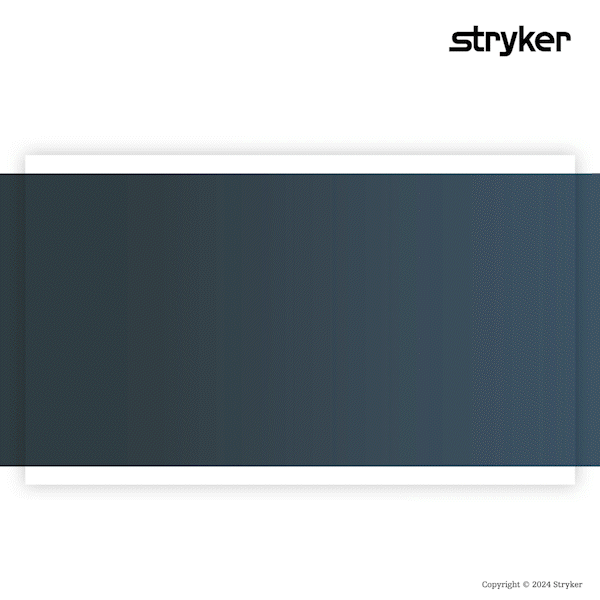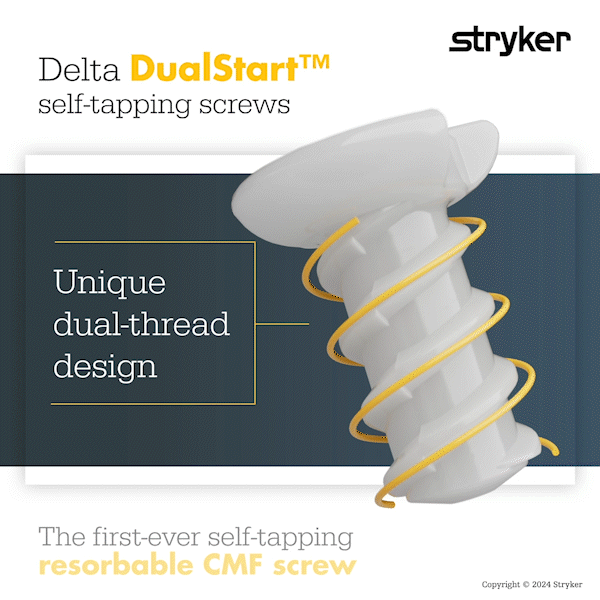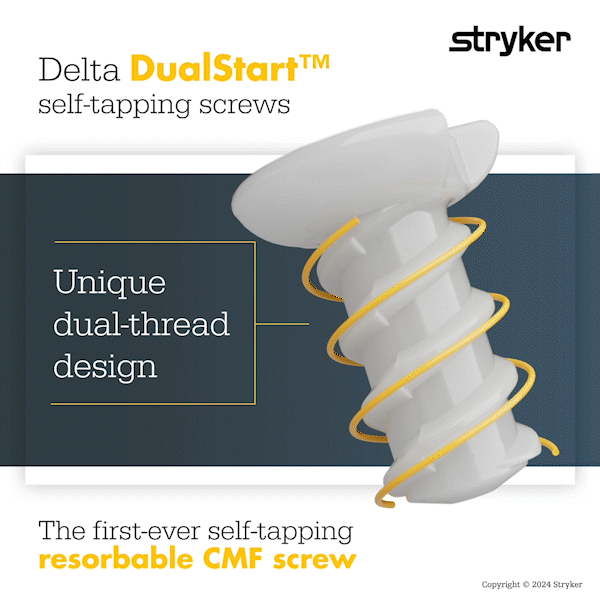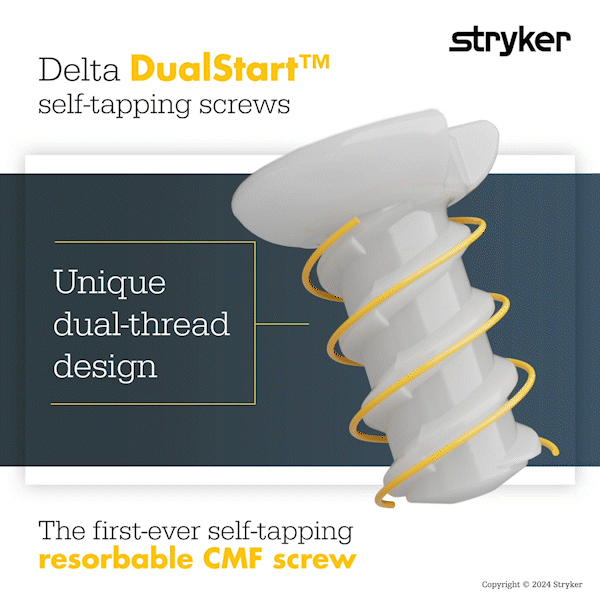
Delta DualStart is the first-ever self-tapping resorbable CMF screw. The DualStart screw features a double-helix thread, and eliminates the need for a tap to be required.
Design modifications allow for:



Delta System Overview
Overview
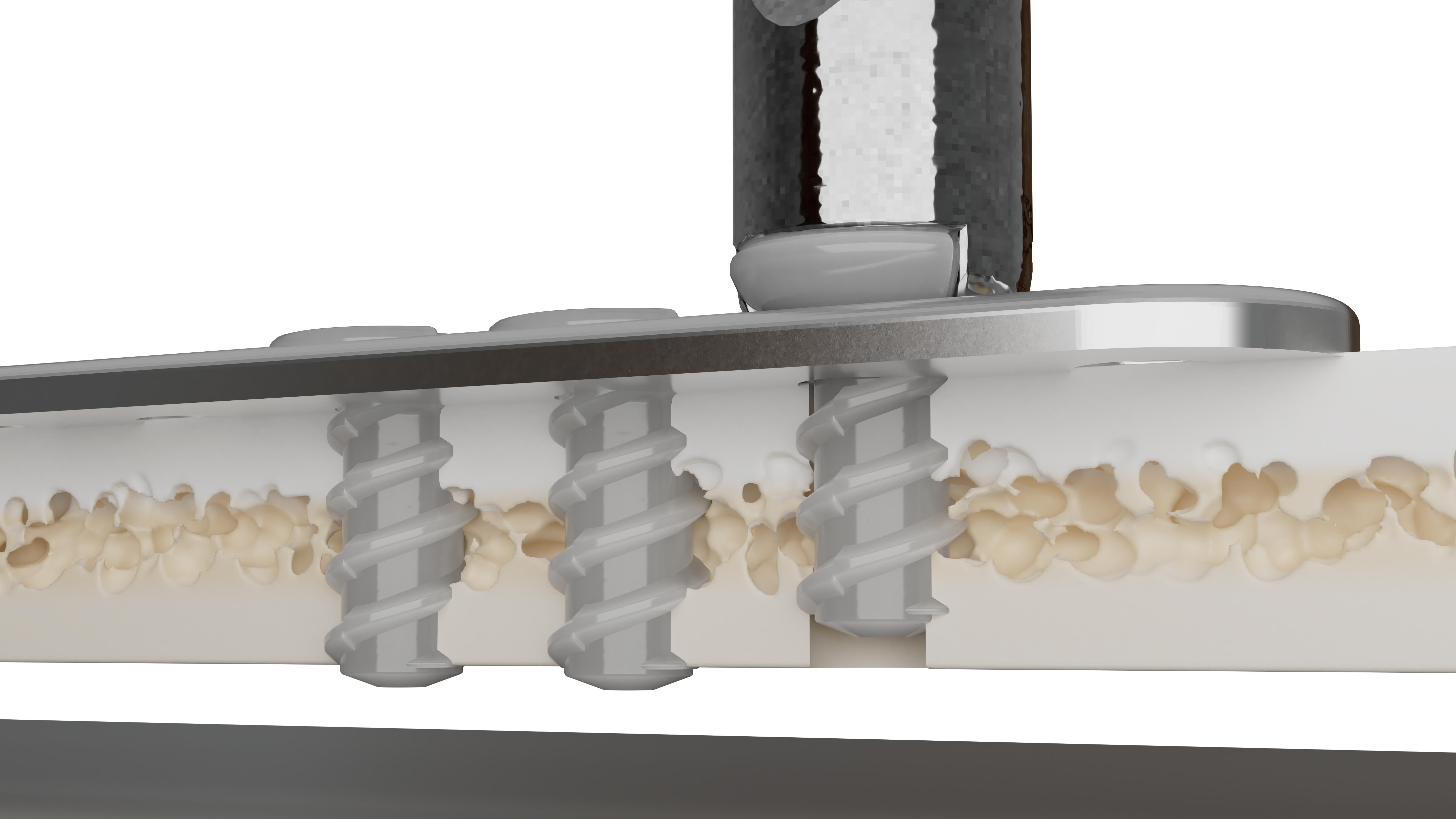
Delta is a resorbable plating system designed for the use in the fixation of bones of the craniofacial, maxillofacial, and midfacial skeleton affected by trauma, or for reconstruction.
Delta has a copolymer composition of poly L-lactide (PLLA), polyglycolide (PGA) and poly D-lactide (PDLA) in a ratio of 85:10:5. It has an attractive combination1 of strength, balanced resorption and contourability.
Features and benefits
Resorption rate
Delta has a gradual resorption time, depending on the patient anatomy and metabolism, of approximately 8-13 months. 2,3
Strength retention
At 12 weeks and 6 months, Delta maintains 85% and 75% of its initial strength respectively1, which can help facilitate structural fixation and reinforcement during osteosynthesis.
Contourability
With the addition of 5% poly D-lactide, Delta plates and mesh are more easily shaped to fit your patients' anatomy.3
Clinical evidence
Abstract
Background
The malar bone represents a strong bone on fragile support and its processes - frontal, orbital, maxillary and zygomatic are frequently the site of fracture. Current study was done to compare the stability of zygomatic complex fracture using Biodegradable plates and titanium miniplates with one point fixation.
Materials and Methods
Twenty patients of zygomatic complex fracture were randomly selected and divided in two groups which were further divided into two subgroups (A, B). Group I patients were treated with titanium miniplate at zygomatic buttress and Group II was treated by bio-resorbable plates. One point fixation was done either at zygomatic buttress or at frontozygomatic suture and it was observed that both the site have been the most favored site of rigid internal fixation in terms of stability, aesthetics and prevention of rotation of the fracture segment in either vertical or horizontal axis.
Conclusion
There is no significant difference in post operative outcomes between two groups, but still bioresorbable system has some advantage over titanium system as these plates resorbs over a period of time and does not cause any interference with growth and post operative radiotherapy. However application of biodegradable system demands highly précised technique.
Abstract
Objective
The aim of this prospective study was to compare the clinical handling of 3 different biodegradable osteosynthesis materials and to determine whether they can be used for the fixation of all types of zygomatic fractures.
Study design
A total of 54 consecutive patients who presented with displaced fractures of the zygomatic bone between October 2001 and May 2003 were randomly allocated to 3 biodegradable material groups for the fixation of the fractures. A titanium fixation system was used as rescue osteosynthesis whenever biodegradable materials failed.
Results
Seventy-one (75.5%) of 94 fracture sites were fixed with biodegradable osteosynthesis; 23 (24.5%) had to be fixed with titanium plates and screws. No statistically significant difference was found between the 3 biodegradable materials with regard to their suitability for zygomatic fracture fixation (P = .16). Nonstable fixation (n = 7) or the need to fix small fragments (n = 16) were the reasons for using the titanium fixation system as rescue osteosynthesis at these sites. Biodegradable materials were most frequently unfeasible for use at the infraorbital rim and in the zygomaticomaxillary/anterior sinus wall area.
Conclusions
It was possible to stabilize 3 of 4 zygomatic fractures with 1.5- or 1.7-mm biodegradable osteosynthesis. Insufficient fracture stabilization, especially at the infraorbital rim and the zygomaticomaxillary crest/anterior sinus wall, was the main reason to switch to titanium osteosynthesis. The biodegradable screw design is possibly too bulky for these particular bony structures.
Abstract
Abstract
CMF-WC-57_Rev. None_18996
References:
-
Posnick, J.C., and Ricalde, P.: Degradation Rate of Delta (Resorbable) Internal Fixation: Report of 2 Cases, 2004.
-
Ignatius, A.A. and Claes, L.E.: In Vitro Biocompatibility of Bioresorbable Polymers: Poly (L, DL-lactide) and Poly (L-lactide-co-glycolide), 1995.
-
Losken, H.W., Aalst, J.A., Mooney, M.P., Godfrey, V.L., Burt, T., Teotia, S., Dean, S.B., Moss, J.R. and Rahbar, R.: Biodegradation of Inion Fast-Absorbing Biodegradable Plates and Screws, 2008.
CMF-DELT-SYK-947830_REV-0